In those trials the Pfizer-BioNTech vaccine was 52 percent effective after one dose and 95 percent effective after two doses. Based on the evidence so far the new variants of SARS-CoV-2 including the B117 and the 501YV2 do not alter the effectiveness of the Moderna mRNA vaccine.
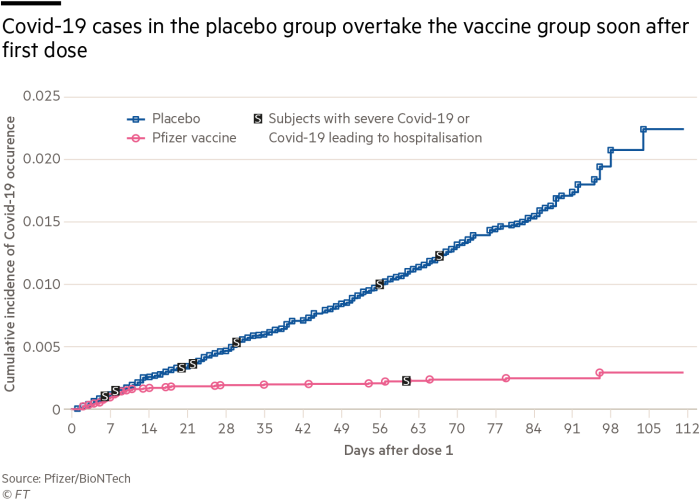 Pfizer Biontech Vaccine Starts Working 10 Days After First Dose Says Fda Financial Times
Pfizer Biontech Vaccine Starts Working 10 Days After First Dose Says Fda Financial Times
This onset of soreness was quite faster than when I received my first dose.

Moderna after first dose. For days 15-28 or up to the first week after the second dose protection from the first dose was estimated at 91. The range for this was between 74 and 97. These reactions included pain redness swelling and itching at the injection site.
Got my first moderna shot at CVS on Wednesday. Learn about common side effects of COVID-19 vaccines and when to call a doctor. Or after getting the first dose of the vaccine you should not get a second dose of either of the mRNA COVID-19 vaccines.
HealthDayTheres some sobering news for the millions of Americans who. Describes the experiences of four people who developed COVID arm 710 days after receiving the first dose of the Moderna vaccine. The Centers for Disease Control and Prevention reported last week that the first doses of the Pfizer and the Moderna vaccines were 80 percent.
REUTERSDado RuvicIllustration COVID-19 vaccines developed by Pfizer Inc with BioNTech SE and Moderna Inc reduced risk of infection by. Does it work against new variants. In the US the Centers for Disease Control and Prevention has recommended giving second doses of Pfizers vaccine 21 days after the first and 28 days after the first for Moderna with an interval.
Modernas coronavirus vaccine may cause alarming delayed side effects from its first dose among a portion of recipients. A second dose would not be expected to confer immunity within that time. Unlike Johnson Johnsons vaccine which requires one dose Pfizers and Modernas vaccines require two shots given three to four weeks apart.
People want to know if they can travel after getting the first dose of a Pfizer or Moderna vaccine. For days 15-28 or up to the first week after the second dose protection from the first dose was estimated at 91. For the Moderna vaccine clinical trials that vaccine was 508 percent.
An expert told Insider that its relatively safe for partially vaccinated people to travel. But I am glad I decided to go. I was very nervous about getting the vaccine I had an appt to get it before Wednesday and didnt go.
Both the Pfizer-BioNTech and Moderna vaccines appear to provide some protection after the first dose. But their headline efficacy ratings are. Both doses should be Moderna COVID-19 vaccine.
In exceptional situations if the vaccine product given as the first dose cannot be determined or is no longer available any mRNA COVID-19 vaccine product may be administered at least 28 days after the first dose. So Im hoping my 2nd. An allergic reaction is considered severe when a person needs to be treated with epinephrine or EpiPen or if they must go to the hospital.
A study from the CDC reports the first dose of Pfizer or Moderna vaccines is about 80 percent effective. My shoulder was sore for about three days after the first shot but this time it was only sore for a few hours. After the first dose of the vaccine 74 percent of Moderna recipients reported an injection site reaction.
Some who received their first shot of the Moderna. The Moderna vaccine has been shown to have an efficacy of approximately 92 per cent in protecting against COVID-19 starting 14 days after the first dose. The range for this was between 74 and 97.
Recipients of both the Moderna and Pfizer mRNA vaccines commonly reported pain at the injection site and redness after the first dose along with fatigue and joint pain after the second dose. 64 at first dose 94 at second. Every effort should be made to determine which vaccine product was received as the first dose.
The second dose pushes effectiveness up to 90. PfizerModerna vaccine protection. The authors of the report note that COVID arm is uncommon not.
Had the moderna jab waited 15 minutes for observation not a problem left after 15 minutes and I swear I was super surprised I didnt have 1 side effect not even a sore arm where they jabbed me.